Biovian chooses Elomatic to design ATMP cleanrooms in 6,400 sqm facility
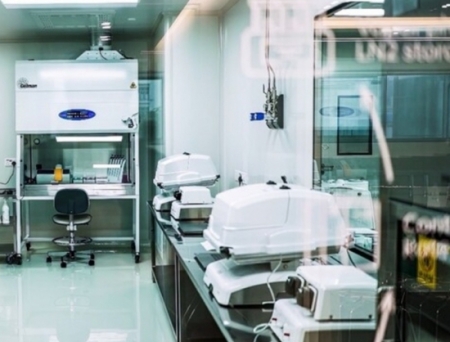
Biovian Oy, a contract development and manufacturing organisation (CDMO) specialising in biopharmaceuticals, has announced a major investment of over €50 million ($55m) to expand its manufacturing facility in Turku, Finland.
The CDMO has chosen Elomatic to design the cleanrooms and implement the space for the new Biovian Oy manufacturing facility.
"We are proud to be part of Biovian's big investment and work together with the skilled local partners involved. Together we will build a new production facility to support Biovian's mission," said Mona Åkerholm, Senior VP of Pharma at Elomatic.
The expansion project is expected to be completed in December 2024, with the new facility fully operational by 2025.
The new facility will cover an area of 6,400 sqm (69,000 sqft), and house cutting-edge equipment and advanced technologies to support the development, manufacturing, and testing of ATMP (Advanced Therapy Medicinal Products), such as adenoviral and AAV (adeno-associated viral) therapies. It will also feature dedicated Class A to D cleanroom areas for bulk drug substances as well as final drug product manufacture.
The expanded facility will adhere to current good manufacturing practices (cGMP) and fulfilling both EU and US GMP requirements ensuring the delivery of safe, reliable, and compliant biopharmaceutical products.
The investment is a strategic move aimed at meeting the growing demand for high-quality biopharmaceutical manufacturing services and further solidifying Biovian’s position as a trusted partner in the biopharmaceutical industry. Together with Biovian’s existing manufacturing facility, the new premises will increase the total area up to 11 500 sqm (123 800 sqft). The additional space complements Biovian’s existing manufacturing facilities in the area and will enable Biovian to undertake larger-scale viral vector as well as microbial protein projects and concurrent manufacturing campaigns by increasing overall production capacity and flexibility.
The expansion project is expected to be completed in December 2024, with the new facility fully operational by 2025. The investment is expected to create 100 job opportunities in the region, adding to Biovian’s already approximately 200 strong team.
Antti Nieminen, CEO of Biovian, expressed his enthusiasm about the new manufacturing facility: “The new manufacturing facility will not only strengthen Biovian’s position as a key player in the CDMO sector, but also contribute to the growth of the biopharmaceutical industry in Finland and most of all provide vital therapies to patients suffering from today’s untreatable diseases."
Last news about this category
We use our own and third party cookies to produce statistical information and show you personalized advertising by analyzing your browsing, according to our COOKIES POLICY. If you continue visiting our Site, you accept its use.
More information: Privacy Policy















