HR Pharmaceuticals Secures Exclusive Rights for Duette Dual Balloon Catheter
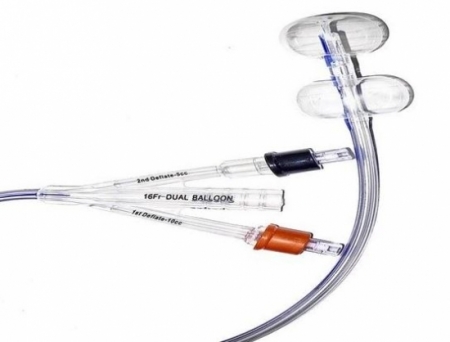
HR Pharmaceuticals, Inc. (HRP) has announced a groundbreaking development in the field of urological care with its exclusive commercial agreement with Poiesis Medical LLC.
This strategic collaboration grants HRP exclusive commercialization rights in North America for Poiesis's revolutionary Dual Balloon Catheter, known as Duette™.
The agreement positions HRP to expand its growing urological portfolio, offering a unique solution to address challenges associated with Foley catheterization, particularly crucial for specific patient populations. The Duette™ Dual Balloon Catheter boasts a novel design that has demonstrated significant benefits compared to traditional Foley catheters, promising improved patient outcomes.
Colby Wiesman, President of HR Pharmaceuticals, Inc., expressed confidence in the potential of the Dual Balloon Catheter, stating, "This product will become an important part of our broader urology strategy over the next 12 months. We see a great opportunity, especially for patients requiring indwelling catheterization greater than five days."
The innovative technology behind the Dual Balloon Catheter is credited to Dr. Bruce Wiita, a practicing urologist and decorated Vietnam veteran, as highlighted by Gregory Wiita, CEO and Founder at Poiesis Medical LLC. "The technology was born out of a genuine desire to improve the quality of life for patients enduring prolonged catheterization and to alleviate the strains it places on the healthcare system," he shared.
HRP is set to commence processing North American orders for Duette Dual Balloon Catheters from January 2, 2024. The product will be available through major national distributors, including Cardinal Health, McKesson, Medline, and Owens & Minor. The collaboration signifies a new chapter for both companies, with the potential to make a meaningful impact on patient care.
Last news about this category
We use our own and third party cookies to produce statistical information and show you personalized advertising by analyzing your browsing, according to our COOKIES POLICY. If you continue visiting our Site, you accept its use.
More information: Privacy Policy

















