Innoblative Wins FDA IDE Approval for SIRA Device, Advancing Breast Cancer Surgery Innovation
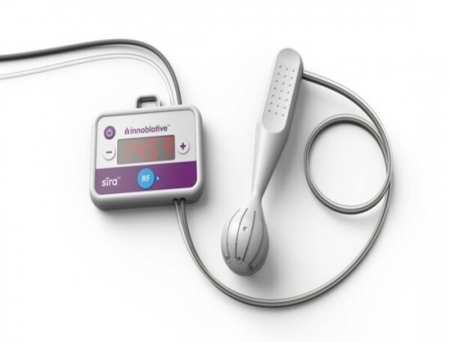
Innoblative Designs, Inc., a private medical device company addressing clinical unmet needs for patients with breast cancer has announced that the US Food and Drug Administration has approved its Investigational Device Exemption (IDE) application, paving the way for the company to initiate its US feasibility study. The study will evaluate the safety and effectiveness of the company's SIRA® RFA Electrosurgical Device in patients undergoing breast-conservation surgery (BCS).
"The SIRA device, specifically designed as an adjunct to BCS, addresses residual cancer in surrounding tissue at the time of the initial lumpectomy procedure. The technology provides a more palatable option for patients and aims to eliminate the need for subsequent radiation therapy or reoperations," commented Richard Stark, CEO of Innoblative.
"We are thrilled to have secured IDE approval, a significant milestone for the company and a testament to the team's hard work and dedication. We look forward to initiating the US feasibility study to further validate our technology, which we believe will be a game-changer for breast cancer patients," added Stark.
Innoblative's SIRA device is a single-use, disposable applicator built for intraoperative ablation of the post-lumpectomy cavity during breast conserving surgery. With a unique spherical design, SIRA circumferentially delivers radiofrequency (RF) energy and yields reproducible ablation depths to the entire cavity. This provides greater confidence that the margins, where residual cancer may reside, have been treated. RF ablation has been shown in multiple long-term clinical studies to reduce reoperations and may reduce local recurrence in breast cancer treatment.
Currently, there are no RF devices specifically designed for breast cancer. Conventional RF systems are not optimised for treating lumpectomy cavities, often resulting in inconsistent treatment depths and ablations. The SIRA device aims to provide a reduction in positive margin rates, reduce BCS reoperations, and offer a one-time non-ionising therapeutic alternative to radiation therapy in select patients, with the potential to reduce healthcare costs and improve patient care.
Breast cancer is a significant global health concern with documented impact in all countries. Once diagnosed, patients are faced with difficult decisions regarding available treatment options. Breast conservation therapy (BCT), which includes breast-conserving surgery (BCS) plus radiation therapy, may be recommended.
However, BCS is associated with a high rate of re-operation, with approximately one-in-five patients requiring a follow-up procedure within weeks of the initial excision to address residual cancer. In addition, subsequent radiation therapy can be difficult with frequent treatments over the course of several weeks or months, and the potential for a range of unpleasant side effects.
Innoblative aims to provide an alternative treatment option that allows patients to avoid these concerns and alleviate fears of future recurrences. The SIRA® Device is investigational for use in BCS in the United States.
Breast cancer Innoblative Investigational Device Exemption SIRA Device SIRA RFA Electrosurgical Device US Food and Drug Administration USFDA
Last news about this category


We use our own and third party cookies to produce statistical information and show you personalized advertising by analyzing your browsing, according to our COOKIES POLICY. If you continue visiting our Site, you accept its use.
More information: Privacy Policy














