Medline UNITE Earns FDA 510(k) Clearance for REFLEX HYBRID Nitinol Implant System
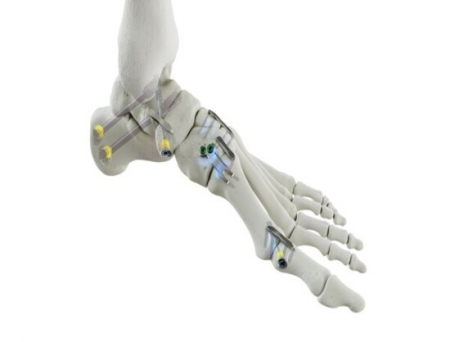
Medline UNITE has received 510(k) clearance from the US Food and Drug Administration (FDA) for its latest implant family, the REFLEX® HYBRID Nitinol Implant System.
While nitinol staples have steadily risen in popularity, static plates and screws are widely used in conjunction with staples to provide added fixation and stability. A key disadvantage of such hybrid constructs is the inherent neutralisation of compression caused by the addition of static devices such as locking plates and cannulated screws.
REFLEX HYBRID combines the compression of a nitinol staple with the stability of a locking plate, offering indication-specific implants designed for MTP fusions and Lapidus procedures. When paired with a cannulated screw with a Dynamic Disc, or a REFLEX nitinol staple, the construct provides dynamic biplanar compression and fixation for a fully dynamic construct.
"REFLEX HYBRID further demonstrates our commitment to offering innovative solutions for foot and ankle surgeons. The first to market product addresses gaps in the current competitive landscape, including offering indication specific designs, intraoperative compression, and intraoperative adjustment with a nitinol implant," said Scott Goldstein, vice president of product management for Medline UNITE.
The system includes an innovative inserter that allows the surgeon to expand the implant's legs for a streamlined technique. Because both of the implant's legs are on the same side of the joint, the surgeon has the flexibility to adjust the placement of the implant, which is not possible with other nitinol solutions on the market.
"In addition to speed and efficiency, many surgeons have adopted nitinol staples because they provide dynamic compression after implantation and throughout the healing phase. However, staples do not allow the surgeon to achieve compression at the joint prior to deployment of the staple," said Natan Pheil, director of product development for Medline UNITE.
" With REFLEX HYBRID, the surgeon can drill eccentrically through the implant's compression ramp and place a non-locking screw to gain additional compression at the joint before deploying the compressive power of the nitinol implant," added Pheil.
"Biomechanical testing results showed using a HYBRID implant with a screw provides a statistically significant better load to failure and gapping at failure result than a traditional titanium plate and screw construct. Historically, nitinol constructs have not performed better than traditional plate and screw constructs with such testing," said Jonathon Backus, MD of the Steadman Clinic in Vail, CO.
Dr. Backus conducted extensive testing comparing a REFLEX HYBRID and cannulated screw construct to traditional titanium plate and screw, and nitinol staple and screw constructs.
Last news about this category

We use our own and third party cookies to produce statistical information and show you personalized advertising by analyzing your browsing, according to our COOKIES POLICY. If you continue visiting our Site, you accept its use.
More information: Privacy Policy













