Sanoculis Receives CE Mark Approval for MINT Glaucoma Surgery Device in EU
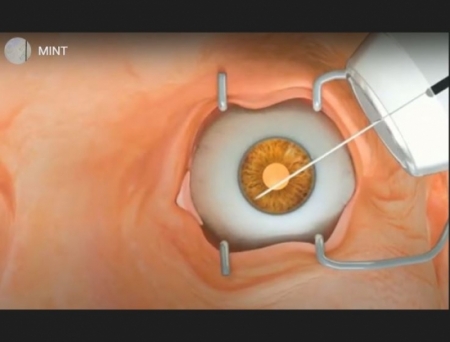
Sanoculis Ltd. announced that it has received CE Mark approval under the Medical Device Regulation (MDR) in the European Union (EU) for its MINT® (Minimally Invasive Nasal Trabeculostomy) product—an innovative, stent-free technology platform for the treatment of adult patients undergoing glaucoma angle surgery.
MINT® utilizes a unique mechanical, semi-automated trephination technology with a cutting-edge diameter of 0.14 mm to create precise openings in the pigmented trabecular meshwork. This stent-free approach is designed to elevate the standard of care by offering a less invasive, more effective treatment option for glaucoma patients.
"MINT® represents a substantial transformation in the field of Minimally Invasive Glaucoma Surgery (MIGS), with significant potential to improve patient outcomes and elevate the standard of care," said Nir Israeli, Co-founder and CEO of Sanoculis. "Clinical data from our prospective, single-arm study with a two-year follow-up indicate that MINT® is both safe and effective in lowering intraocular pressure (IOP), while also reducing the need for glaucoma medications."
Sanoculis plans a selective commercial pre-launch rollout of the MINT® technology later this year.
Last news about this category
We use our own and third party cookies to produce statistical information and show you personalized advertising by analyzing your browsing, according to our COOKIES POLICY. If you continue visiting our Site, you accept its use.
More information: Privacy Policy














