Single Pass Receives FDA Clearance for Kronos Biopsy Closure Device
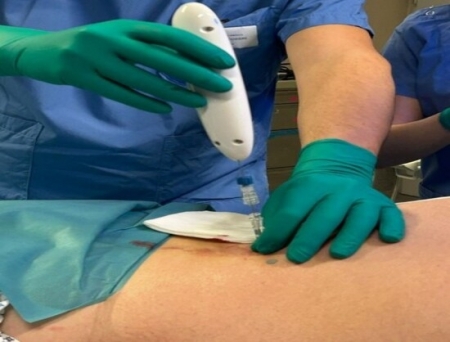
Single Pass, Inc. has announced the US Food and Drug Administration (FDA) clearance for its Class II Kronos biopsy closure device, marking a significant milestone in advancing medical technology.
Under the leadership of Bill Colone and the Board of Directors, the Single Pass team collaborated with their contract manufacturing partner, M4D, located in Lake Forest, CA, to bring the Kronos device to market. With prior achievement of the CE Mark under the EU MDR regulations, the Kronos device is now commercially available globally.
"We are thrilled to have received FDA clearance for our Kronos biopsy closure device," stated Bill Colone, Co-founder, and CEO of Single Pass. "The Kronos device represents a significant advancement, offering healthcare professionals a reliable and efficient solution for post-biopsy bleeding and improved patient care," added Colone.
Mermaid Medical, the US distribution partner, stands ready to launch sales in the US, ensuring immediate national access to this groundbreaking technology. This strategic partnership aims to facilitate widespread coverage for this innovative and unique technology, ultimately benefiting patients and healthcare providers across the country.
Last news about this category

We use our own and third party cookies to produce statistical information and show you personalized advertising by analyzing your browsing, according to our COOKIES POLICY. If you continue visiting our Site, you accept its use.
More information: Privacy Policy
















