Terumo Neuro Secures FDA Premarket Approval for Carotid Stent System
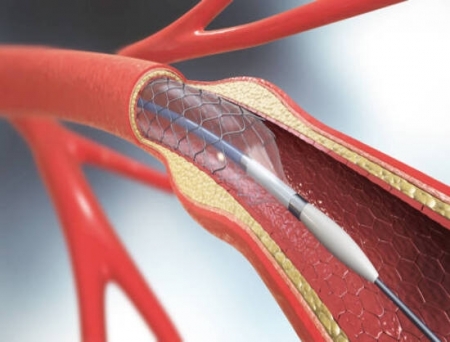
Terumo Neuro, a company in neurovascular innovation and a wholly owned subsidiary of Terumo Corporation, has announced that its Carotid Stent System has received Premarket Approval (PMA) from the US Food and Drug Administration (FDA).
This milestone marks the first dual-layer micromesh carotid stent approved in the United States, offering physicians a clinically proven option to improve patient outcomes in carotid artery disease treatment.
Terumo Neuro's Carotid Stent System is indicated for the treatment of carotid artery stenosis in patients at increased risk for adverse events following carotid endarterectomy.
The device is intended to treat patients with de novo atherosclerotic or post-endarterectomy restenotic lesions in the internal carotid arteries or at the carotid bifurcation, with ≥50 percent stenosis in symptomatic patients or ≥80 percent stenosis in asymptomatic patients, as determined by angiography. The device accommodates vessel reference diameters between 3.5 mm and 9.0 mm at the target lesion.
Last news about this category
We use our own and third party cookies to produce statistical information and show you personalized advertising by analyzing your browsing, according to our COOKIES POLICY. If you continue visiting our Site, you accept its use.
More information: Privacy Policy














